Introduction
As a general manufacturer of dental care products, GC takes the ordinary people’s perspective, providing true health to the people of the world. GC products are made with the core values of safety and effectiveness, in line with the company’s ethos of putting quality first, and we believe that protecting the global environment is a major part of our mission. We monitor each stage of a product’s life, testing for safety and for the impact of our products on resources and the environment. We scrutinize the production process to ensure that we are conserving energy, conserving resources and minimizing packaging waste. We examine the use of our products to ensure that they are energy saving and economical in terms of the resources they consume, and we examine disposal methods for our products. Naturally, GC seeks to minimize any adverse effects, paying maximum attention to the issue of the environment from the time each product is developed until its use by the consumer. As a result of GC’s endeavors in this respect, the company earned ISO 9001 Quality Systems certification in 1994, and ISO 14001 Environmental Management System certification in 1998. GC topped this in 2000 by becoming the first company in the dental industry to be awarded the Deming Application Prize. In 2003, GC was certified for ISO 13485, and in 2004, GC became only the 18th company in the world to have won the Japan Quality Medal, the pinnacle of quality control. As a further mark of the company’s reputation, the global marketplace has come to recognize GC’s products as being synonymous with quality and eco-friendliness. With an outstanding range of some 600 different excellent product types marketed in over 100 countries, GC is helping to maintain the health of people the world over. Given the importance of predicting and supporting different market demands from one region to the next, GC’s approach is to site its manufacturing operations in the optimum location for each market. Naturally, the company’s three main manufacturing bases around the world are all ISO-certified and deliver products of the highest quality.
People Friendly and Environment Friendly
Through dentists, technicians, hygienists and other dental professionals around the world, GC's quality dental care products make a major contribution to people's health. As a dental product manufacturer of global standing, GC Corporation constantly seeks to produce original products that are environmentally friendly as well as patient friendly and going a step ahead of patient's needs, while at the same time taking great care to minimize the impact of manufacturing processes on the environment.
Producing products that are people friendly and earth friendly, we will continue to create highly value-added products so as to provide oral health to the people of the world during the 21st century, a century we regard as the century of health.
ISO Certification, The Deming Application Prize and The Japan Quality Medal
As a general manufacturer of dental care products, GC takes the ordinary people's perspective, providing true health to the people of the world. GC products are made with the core values of safety and effectiveness, in line with the company's ethos of putting quality first, and we believe that protecting the global environment is a major part of our mission. We monitor each stage of a product's life, testing for safety and for the impact of our products on resources and the environment. We scrutinize the production process to ensure that we are conserving energy, conserving resources and minimizing packaging waste. We examine the use of our products to ensure that they are energy saving and economical in terms of the resources they consume, and we examine disposal methods for our products.
Naturally, GC seeks to minimize any adverse effects, paying maximum attention to the issue of the environment from the time each product is developed until its use by the consumer. As a result of GC's endeavors in this respect, the company earned ISO 9001 Quality Systems certification in 1994, and ISO 14001 Environmental Management System certification in 1998. GC topped this in 2000 by becoming the first company in the dental industry to be awarded the Deming Application Prize. In 2003, GC was certified for ISO 13485, and in 2004, GC became only the 18th company in the world to have won the Japan Quality Medal, the pinnacle of quality control. As a further mark of the company's reputation, the global marketplace has come to recognize GC's products as being synonymous with quality and eco-friendliness.
With an outstanding range of some 600 different excellent product types marketed in over 100 countries, GC is helping to maintain the health of people the world over. Given the importance of prediting and supporting different market demands from one region to the next, GC's approach is to site its manufacturing operations in the optimum location for each market. Naturally, the company's three main manufacturing bases around the world are all ISO-certified and deliver products of the highest quality.
History
1921 |
|
1922 |
|
1925 |
|
1927 |
|
1934 |
|
1938 |
|
1941 |
|
1946 |
|
1948 |
|
1951 |
|
1953 |
|
1956 |
|
1957 |
|
1959 |
|
1965 |
|
1966 |
|
1969 |
|
1971 |
|
1972 |
|
1973 |
|
1974 |
|
1976 |
|
1977 |
|
1978 |
|
| 1981 |
|
1982 |
|
1983 |
|
1984 |
|
1989 |
|
1990 |
|
| 1991 |
|
| 1992 |
|
1993 |
|
| 1994 |
|
| 1995 |
|
1996 |
|
1998 |
|
1999 |
|
2000 |
|
2001 |
|
2002 |
|
| 2003 |
|
2004 |
|
| 2005 |
|
| 2006 |
|
| 2007 |
|
2008 |
|
| 2009 |
|
2010 |
|
| 2011 |
|
2012 |
|
| 2013 |
|
Message From Management

GC Corporation Sees The 21st Century as the "Century of Health" and is Striving to be the World's #1 Manufacturer of Dental Care Products That Support the Vitality and Well-Being of People All Over the World
Dental Care, which covers both Oral and Dental Health, directly helps improve quality of life. As a type of health care that supports people's vitality and well-being, Dental Care is expected to play an even greater role in the future. GC Corporation is adapting to global changes, such as aging and the increased attention to health, by committing to contribute to the "Century of Health" realization and promoting the creation of a society focused on health and longevity.
In 2011, when we celebrated our 90th Anniversary, we established a new GC Corporate Center in Hongo, Tokyo, moving our headquarter's functions and creating several floors specifically designed for sharing information with our customers. Having designed this site to be a center for the dissemination of useful Dental Health Information, we hope that it will create new value as a space that can be used for communicating with the public regarding various issues related to Dental Care.
In September 2013, we opened a space called Kamuller, which aims to propose concepts to help people achieve the keys to health and longevity by chewing properly and enjoy eating. We also have the GC R&D Center, a research development center that serves as the starting point for our "Communications Loop", GC's space concept and the Fuji Oyama Factory. This center serves the functions of a mother plant for overseas developments (to Belgium, the United States and China) and as a manufacturing base for the GC Group. To promote the organic integration of our three major basic functions in Japan and multinationalism in the sense of strengthening our activities in the global market by adapting them to local needs and characteristics, we have established GC International AG in Switzerland as a new platform that will lead our activities in Europe, the United States and Asia.
Our worldwide colleagues at these global centers will all be working towards the shared goal of achieving Vision 2021, whose target is our upcoming 100th Anniversary, by putting GQM2021 into practice. Together, we are striving to make a company that delivers health and strong smiles to people all around the world.
We at GC Corporation are sincerely grateful to everyone who has supported this company over the 90 years since its founding. We hope you will continue to work with us in our efforts to create a dental industry that has immense future potential.
Relationship with Our Customers
With Dental Professionals
GC maintains close communication with clinicians, researchers and other professional practitioners of dental medicine. As part of this communication process we study, develop and provide samples of safer, better products while exchanging the latest academic and clinical information regarding dental care. We will continue to maintain close communication with dental professionals and thereby contribute to the development of dental products.
GC Membership Society
The "GC Membership Society," founded in 1956, is a member organization that provides information to dentists and dental technicians. The idea is to allow dental clinicians to share information with the dental material manufacturer and thus facilitate the development of new products and technologies based on our firmly held view that "in the new era, product research must be done hand in hand with the intended users of these products."
The GC Membership Society has grown from a circle of approximately 3,600 members to a large organization boasting around 43,000 members in 2007. Approximately 47% of all practicing dentists in Japan are members. Last year, in celebration of the GC Membership Society's fifty-first anniversary, we launched a new member organization for dental hygienists. With the organization we can interact with all dental professionals. We actively provide support for members regarding dental care, such as information on new products (brochures of new products before their release in the general market, product discount vouchers and quarterly-issued member journal); academic information (academic seminars, symposiums, GC Membership communities, and education on clinical techniques and dental clinic staff (GC Membership Society seminars).
The "GC Membership Society's Member Site" is a member-only site that features information, tools, materials and other contents that are useful in the clinical scene. The content can be viewed, practiced and utilized by members. The ""GC Membership Society Internet News," which provides the latest academic information and product information in real time, is today accessed by nearly 10,000 members.
Second International Dental Symposium
Following the inaugural "International Dental Symposium" held in 1996 to commemorate the fortieth anniversary of the GC Membership Society and the seventy-fifth anniversary of the company, in February 2006 we organized the "Second International Dental Symposium in Commemoration of the Fiftieth Anniversary of the GC Membership Society" at Tokyo International Forum in Yurakucho. The two-day event was attended by a total of 14,965 dentists, dental technicians, dental hygienists, dental clinic staff and the general public.
The theme of the Second International Dental Symposium was "Future Prospects of Patient/Citizen-Centered Dentistry." At the opening ceremony, President Makoto Nakao said, "Believing in health and longevity supported by occlusion and mastication, and also in the roles played by eating and communication-two basic activities of man - as well as our physiological functions, we aspire to make the twenty-first century the 'Century of Health' by working together with dental professionals." Thereafter, Chairman Michéle Aerden of the FDI (FDI World Dental Federation) and President Takayuki Kuroda of the IADR (International Association for Dental Research) gave their messages.
At the GC Dental Show held concurrently with the symposium, we exhibited new products and conducted demonstrations in a manner allowing "visitors to take their time and thoroughly examine our new offerings and the hot products." We set up trial booths for brushing, mouth-washing and implantation, etc. We also held a seminar entitled "The Imaging Diagnosis of Implantation" and others. The show attracted approximately 3,800 visitors.
Third Citizen's Dental Awareness Survey
Following the first and second surveys in 2004 and 2005, we conducted a third survey to shed light on the awareness, requests and other comments that citizens have regarding dental care. We randomly sampled men and women of ages 15 through 79 in 47 prefectures across Japan, and asked questions by focusing on the relationships between "age, sex, postal code and other social factors" and "visit to a dentist and choice of paying by insurance or privately." We received replies from approximately 1,200 respondents. The tabulated results are presented as reference to member dentists of the "GC Membership Society."
MI Concept to Improve QOL of All People
At GC we instituted our unique MI (Minimal Intervention) concept in 2000 by rephrasing the original MI concept proposed by the FDI in 1999 and defining the three approaches of "Identify," "Prevention" and "Treatment & Control" to better fit the clinical situation in Japan. Since then, GC's MI concept has been at the basis of various products and information developed and disseminated by the company. Today, our MI concept is recognized in the dental community as an ideal framework for programs designed to treat dental caries. We are developing MI-related products to help all people maintain and improve their quality of life (QOL).
GC is also involved in the development of a full range of denture products and care products intended to improve the QOL for senior citizens, whose population is growing in today's super-aging society.
"MI21.NET" Website
With the General Public
With help from dental professionals, GC provides the general public with useful information to encourage dental and oral health. Our goal is to foster and entrench a new mindset for oral care so that all people can live in health and happiness.
"Citizen's Forum on Dental and Oral Health"
In February 2006 we co-sponsored four sessions of the Citizen's Forum on Dental and Oral Health (organized by the Tokyo Dental Association, with GC as a special sponsor) over two days during the Second International Dental Symposium as health education events with public participation.
During the forum, participants were given useful information on dental and oral health through fun-filled programs, including a healthy exercise session with instructor Hiromichi Sato, who is hugely popular among children for his appearance in a children's exercise program, and a tooth-brushing lesson by Dr. Nanae Kuraji, a dentist. The venue was packed to full capacity each day by about 2,800 parents and children.
Dental Information Booklet "Healthy Diamond" (In Japanese Only)
In collaboration with Diamond Inc., the publisher of the business magazine Diamond Weekly, which provides economic, financial and corporate information, we published an information booklet for the general public called "Healthy Diamond." Two issues of the booklet were sent to subscribers of Diamond Weekly. The compact booklets are filled with information people should know about their teeth, with all topics explained in plain language in a manner anyone could understand. In the first issue, entitled "Periodontal Diseases and Health of the Whole Body" (December 2006), and the second issue entitled "The Importance of a Proper Bite" (September 2007), renowned dentists in their respective fields explain the relevant information in an easy-to-understand manner.
"Information on Dental and Oral Health" Page For Citizens (In Japanese Only)
In 2006 we created the "Information on Dental and Oral Health" webpage on our Internet site as a visual source of information for the entire family. The page features information on periodontal diseases and implants, the correct way to brush your teeth, etc., in order to help the general public gain the correct understanding of dental and oral hygiene, and to foster and entrench a new mindset for oral health in Japan.
We also provide useful contents on the "Cavities" page, which is designed for use at home and primary schools as an educational material. The segment "Cavity Prevention for Parents and Children" talks about the characteristics of cavities in children, brushing technique for small children and other dental care information for parents. It also features games, visual storybooks and other interesting ideas designed to help children learn in a fun setting.
Brushing Class
GC collaborates with local dental associations to participate in citizen's events, and also to visit schools and companies on a regular basis in order to give brushing classes and organize other educational programs.
With Dealers
GC products are delivered to dental professionals through the hands of special agents and dealers. So that our products will be used properly, we provide dealers with the correct medical techniques and detailed product information and also ask them to obtain feedback from customers.
Dental College
"Dental College" is GC's original educational framework for dealers. The college consists of seminars to learn the basics of our products, as well as group training programs to support sales representatives. The group training program, held in Tokyo in 2004, had the participation of approximately 150 representatives from our special agents throughout Japan. In a special lecture organized as part of the program, the participants listened to case studies of successfully managed dental clinics, methods of instructing members of an organization, etc. At the group training program for dealers in 2006, over 100 participants attended a lecture entitled "Defensive and Offensive Selling" and took part in sales workshops. We also launched an e-learning site in July 2006. The number of registered users has already reached 310.
Consulting Sales Academy
We at GC want to answer the various requests (on esthetics, functionality, comfort, economy, etc.) from patients and meet the desires of dental clinics to improve the quality of life for their patients. As part of the effort, we have organized the "Consulting Sales Academy" as a workshop curriculum to teach sales representatives the techniques of selling by providing solutions. The event gives dealers an opportunity to learn the management of dental clinics in order to meet the needs of patients.
We conducted a total of six Consulting Sales Academy events totaling more than 40 hours in February through November of 2001 (phase 1), which were attended by approximately 30 sales representatives from special agents across Japan. Inviting dental management consultants as lecturers, the academy touched on wide-ranging topics such as useful information on dental clinic management, management simulation, how to use business planning software, and practical information on cash-flow management. These workshops are designed to foster sales representatives who can also work as consultants. In 2007 (phase 7), the academy was attended by approximately 700 people.
Company Profile
Company Name |
|
Foundation |
|
Capital |
|
Head Office |
|
President & CEO |
|
Business Segments |
|
Subsidiaries & Sales Offices |
|
Relationship with Society
GC, as a dental manufacturer, considers excellence in its core business to be the most important mission. We will grow into a more trusted company by interacting with society through our core business.
GC Fuji IX
Under a joint research project with the WHO (World Health Organization) on the development of technologies and materials suitable for the dental-treatment environment in developing countries, GC developed a high-strength glass ionomer cement called "GC Fuji IX." GC Fuji IX can be used with a dental procedure known as ART (Atraumatic Restorative Treatment), which does not require special technology or machinery. GC Fuji IX was introduced by the WHO on World Health Day in 1994 as an innovative restorative method that can be implemented in developing countries where complete facilities are not available and the standards of hygiene are low.
The technology has been applied in numerous countries since then. GC Fuji IX is also widely used in industrialized nations as a restorative method in keeping with today's emphasis on retaining the tooth structure. The theme of the 1994 World Health Day was "Oral Health of A Healthy Life." The year 1994 was designated as the "Year of Oral Health", and GC served as an official supporter as a global dental company contributing to the cause of WHO activity. The circle of efforts to enhance oral health is expanding throughout the world.
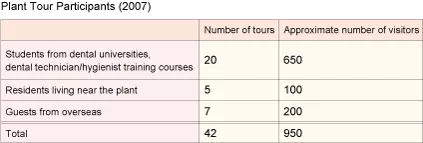
Plant Tour
Our Fuji Oyama Factory offers tours for the dental students and our customers in order to show them first-hand how GC products are made, in hope that it helps deepen their understanding of our advanced technologies and commitment to product quality, and in the process it helps create enthusiastic supporters of the GC name. The plant also communicates with residents in the neighboring community to explain the efforts we are making to reduce the negative impact on the environment and provide opportunities for them to become more interested in their dental and oral health.
Tour guides are selected from among associates, and appointed guides study our products in detail to gain in-depth knowledge so that they can share it with visitors. Additionally, they create tour brochures themselves and strive every day to give better tours.
Achievement of Zero Waste Plant and Cleanup Around Plant
The Fuji Oyama Factory became a "zero waste" plant in September 2007. All waste materials generated from the plant are classified and recycled/reused.
In addition to reducing waste within the facility, the plant is making the neighboring community cleaner by organizing a bi-annual community cleanup event. This year a total of 360 people participated in the cleanup event.
Scholarship Programs
"Toshio Nakao Chair" at University of Leuven in Belgium
In 1999, the "Toshio Nakao Chair" was established at University of Leuven in Belgium. The course, whose title features the name of our former president, is taught by Dr. B. Van Meerbeek, a world-renowned researcher of dental bonding technique, and other talented staff. The seminar is recognized as one of the premier research vehicles in the field of dental preservation and dental material science.
IADR Toshio Nakao Fellowship
The IADR Toshio Nakao Fellowship was established in 1994. The program provides a scholarship of US $15,000 to dental material researchers belonging to the IADR (International Association for Dental Research). As a member of the international business community, GC will contribute to the fostering of people and research programs that help create a better tomorrow.
Team Minus 6%
GC is a member of a national project called "Team Minus 6%", launched to reduce greenhouse gas emissions by 6% and help Japan keep its promise in the Kyoto Protocol, which came into effect in February 2005.
External Recognition and Awards
Awarded the Insignia of Commander of the Crown of Belgium
In 1995, President Makoto Nakao was awarded the Insignia of Commander of the Crown of Belgium from the Kingdom of Belgium. The relationship between GC and Belgium dates back to 1972, when we opened our European branch in Kortrijk. Subsequently, we built a new office and warehouse in Leuven, near the capital Brussels, in 1984. We also constructed our first European plant in Belgium in 1989 and the second European plant in 1994. Our investment in Belgium over the years, and its positive benefits in stimulating the local economy and creating jobs, has been recognized by the country.
Awarded the Health, Labour and Welfare Ministry's Award for Distinguished Services in Pharmaceutical Affairs
President Makoto Nakao was awarded the Health, Labour and Welfare Ministry's Award for Distinguished Services in Pharmaceutical Affairs in 2004. The award was given to President Nakao for his diligent efforts in the field of pharmaceutical affairs and hygiene, for his contribution to public health. A total of 91 individuals were awarded in 2004, but President Nakao was the only one from the dental industry.
Quality
GQM Activity
GC's products are sold in over 100 countries across the world, helping bring true health to people around the world. As a global general dental manufacturer, we will never stop making efforts to develop products that live up to the reputation of "GC the Leader in Quality", and GQM* will always be the basis of these efforts.
GQM: GC's Quality Management
GC declared the introduction of GQC (GC's Quality Control) in 1981, as our unique total quality control initiative echoing the management philosophy "Social Contribution, Quality First, and Sense of Fellowship." This in turn reflects the unchanging founding motto of the company based on the concept of "Semui." In 1995, GQC was expanded into the GQM (GC's Quality Management) activity, centered on the three pillar concepts of "Improve Customer Satisfaction," "Improve Employee Satisfaction" and "Improve Quality of Work", which form the kernel of our business management philosophy.
Also in 2000, we defined the following seven key points of GQM in order to drive various efforts toward quality improvement throughout the company:
- Drive VISION management centered on GQM as the core of the business management approach;
- Implement global strategies based on the basic concept of "globally optimized sourcing";
- Implement product strategies designed to let us develop "the world's best products" and enter into new business fields;
- Realize great production capability that allows us to create "quality" products to meet our customers' needs;
- Implement sales strategies developed through a clear division of the roles of "people who create, sell and use";
- Improve the quality of our business operations through digital management utilizing IT;
- Become a company united by "fellowship," deriving its energy from its "people."
President's Review
The president of GC regularly visits various departments and conducts "President's Review." The purpose of this is for the president to visit various departments and verify with his own eyes how the company's policies are understood and implemented by those departments. Through this practice the president can also determine the degree to which these policies are implemented and whether they are entrenched throughout the organization. Following each review, through a series of meetings the employees and management exchange ideas to identify the issues. The president's review not only helps manage the results of solutions implemented to address the issues but has also become a part of our improvement process.
During each six-month period the president spends a total of about a month touring different departments and interviewing employees on the actual implementation of measures designed to achieve the policies and targets presented by the management, while also answering questions and requests raised by employees. At the end of each president's review session, which lasts from three to six hours, a review report describing the specific areas of improvement and issues to be addressed is prepared. These review reports are used to improve how we view things in a midterm run so that we can raise the standard of our PDCA cycle and thereby contribute to the promotion of GQM.
Fifty of the president's reviews were conducted in 2007.
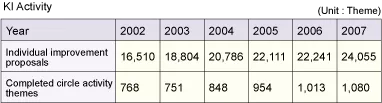
KI Activity and CFT Activity
To "Improve the quality of work," the objective cited as one of the three pillars of GQM, we are driving KI (Kaizen & Innovation) activity. The KI activity is intended to harness synergy among the "fellow employees who respect one another," in keeping with our management philosophy, and thereby promote kaizen (improvement) and innovation. Each department sets its own theme for the improvement of work quality and presents the outcomes of efforts made under these themes, thus fostering a sense of competition that spurs the company-wide effort toward quality improvement. The KI Activity Presentation Conference is held in June and November of each year.
For each issue that is deemed urgent from the viewpoint of corporate management, a CFT (Cross-Functional Team) is formed to gather members with various kinds of experience and skills from multiple departments so that actions can be implemented based on a top-down approach. A number of these cross-functional teams are active throughout the organization, whereby a focused effort is made to resolve issues that affect the entire company.
Receiving the "Deming Application Prize"
GC, in the year 2000, became the first company in the dental industry to receive the "Deming Application Prize".
The Deming Prize (Japanese Administration Office: Union of Japanese Scientists and Engineers) is the highest honor in the area of quality control, and is conferred upon companies (or individuals) that have made a significant improvement in business result through total quality control implemented company-wide. The Deming Application Prize is given annually to the company that has made significant improvements in business results through the application of total quality control.
GC received this coveted prize for its business activity, which is aimed at "becoming a company that improves quality throughout the organization and provides true health for people around the world," and is implemented through GQM to form the basis of the management approach.
Receiving the "Japan Quality Medal"
GC, in 2004 became the 18th company in the world to receive the "Japan Quality Medal," the highest echelon in quality control in Japan.1
The Japan Quality Medal was established to commemorate the first International Conference on Quality Control (ICQC), which was held in Tokyo in October 1969. It is given to a company or business unit each year that has received the Deming Prize and applied TQM2 continuously for three years or more (including the year of conferment of the Deming Prize), and has thereby achieved a remarkable business result.
The reason for GC's winning this prize is as follows: "(GC) has steadily implemented its policy based on a Mid-Term Management Plan to achieve the 2010 VISION initiative, and has achieved steady outcomes in the running of the company through active promotion of excellent quality management efforts, such as the development of new technologies and products, the improvement of productivity under factory innovation programs, the establishment of an onsite quality-assurance system, the innovation of production management systems, the centralization of information using IT, human resource development based on a skills list, quality management, and environmental management."
1. GC Dental Products, an affiliate of GC, received the Japan Quality Medal in 2006 as the twentieth winner of the award. In Europe in the same year, GC Europe received the "Committed to Excellence" prize from the EFQM (European Foundation for Quality Management).
2. TQM (Total Quality Management). GC is implementing its own version of TQM called GQM.
Quality Assurance at GC
GC's most important social responsibility, as a company involved in dental medicine, is to provide products that are better and safer. We are striving to make quality products that will usher in the "Century of Health".
Quality-Assurance System
Dental materials and devices are becoming increasingly advanced and complex. Therefore, it is increasingly important to ensure the quality and reliability of these materials and devices. GC's quality-assurance system is a comprehensive approach that encompasses all the processes relating to the needs of product users, the seeds of GC and the oral health of citizens, as well as the associations among all these processes. Through this system we provide better products and services offering the level of quality required by customers.
QA Certified Section Scheme
To reduce problems in the manufacturing process and raise the standard of quality, in 1992 GC introduced a scheme of certifying the manufacturing sections that met the required quality-assurance level (QA Certified Section Scheme). The scheme has since been expanded to the logistics, customer service, engineering and sales operations of the company, where each section strives to improve its quality-assurance level.
To provide an overview of the certification steps, each section receives an advanced preliminary evaluation and preliminary evaluation by the Quality Assurance Department, as well as a final evaluation by the president and the officer in charge, based on an evaluation check sheet specifying the relevant information regarding the issues addressed by the section. The section that has been certified is given a QA Certificate. Currently, we have 56 certified sections. Certified sections must receive an evaluation every two years in to renew the certification. The introduction of this scheme has produced the following benefits:
- Reduction in percent-defective numbers in processes and manufacturing warranty claims;
- Activation of corrective activities and improvement activities;
- Improvement of process control levels through utilization of control charts, foolproof measures, etc.;
- The fostering of a positive competitive spirit among sections, and acceleration of creative efforts in each section;
- Growth of section chiefs who will shoulder the company in the future;
- Improvement of the overall quality-assurance level throughout the company as a result of expanding the scheme beyond the manufacturing operation to also cover indirect and sales operations;
- Creation of a key selling point in the examination processes for the Deming Prize and Japan Quality Medal, helping the company win both prizes;
- Enhancement of our dedication to customer satisfaction.
In 2007, various sections of our overseas plants were also certified.
Risk Analysis of Products and Gathering of Information from Markets
Products handled by GC are dental materials and devices that affect human life. Accordingly, we pay careful attention to our products by analyzing the associated risks. When developing a new product we conduct analysis and evaluation repeatedly from the design stage, in keeping with the requirements for medical device risk management specified in "ISO 13485." We have a secure information-management system in place, and we endeavor to receive feedback and requests from the dental professionals who use our products.
"ISO 9001" and "ISO 13485" Certification
"ISO 9001" is an international standard for quality management systems encompassing all areas of quality assurance as well as some aspects of organizational management, such as customer satisfaction and improvement. GC, as a global company, became one of the earliest to receive an ISO 9001 certification in 1994. In April 2004 we received the ISO 13485 certification, which specifically provides for the quality assurance of medical devices. GC was the first dental equipment/material manufacturer to receive either certification. Currently, all partner companies of GC are certified under ISO 9001 and ISO 13485.
Information Security
GC received a certification under the JIS Q 15001 (Privacy Mark) standard with the goal of clearly defining our activities to manage information security to protect personal information, and for the purpose of carrying out corporate activities in a manner considering information security. GC Dataland, an information systems management subsidiary of GC, is also certified under the ISO 27001:2006 (Information Security Management System) standard.
Establishment of the "Quality Policy"
We established our quality policy in 1994, and in 2003 portions of the policy were revised.
"Quality Policy"
We will put quality first by focusing on safety and effectiveness while maintaining and improving the effectiveness of our quality management system in order to provide true health for people around the world.
(Explanation)
For people to live in happiness and health every day, it is important that they enjoy the food they eat.
To enjoy the food they eat, we must keep our teeth healthy. The products made by GC must continue, through their roles in dental medicine, to contribute to the dental health of people the world over.
It is our mission to provide better products. We will maintain and improve the effectiveness of our quality-management systems in order to deliver quality products that are not only safe to the human body but also demonstrate the intended effects.
Contribution to the Environment
We at GC strive not only to make highly unique products that meet customer needs, but also to make clean products by giving thorough consideration to the manufacturing process and global environment. We will continue to make environmental efforts around the world so that we can maintain a harmonious coexistence with lives on earth.









Environmental Policy
In 1997, we established our "Environmental Policy" and kicked off full-scale efforts on environmental management. In April 2005 we added explanations to the Environmental Policy, defining specific guidelines by which all employees could help safeguard the environment.
Environmental Policy and Explanations
As a company that provides oral health care, GC Corporation has made promoting environmental management activities one of its top priorities to assure that the Company is in harmony with nature and to have a symbiotic relationship with the local community, and, therefore, has established the following policies.
- Create products that are environment-friendly, promote conservation of resources and energy, reduce waste materials, and maintain and improve the health of the environment through the Company's business activities. "We will develop products that reduce environmental impact by focusing on recycle, reuse, reduction, biomass, etc., save resources and energy (electricity, water, gas, heavy oil, etc.), drive the recycling and utilization of recycled resources, and strive toward resource and energy savings in all aspects of our business based on environmental impact assessment."
- Comply with the relevant environmental regulations, laws and other requirements to which the Company subscribes. "We will observe at the minimum all relevant legal standards, regulations, agreements made with local government bodies, and the requirements set by communities and industry associations regarding the environment."
- Strive to meet the environmental objectives and targets, and review the environmental policy annually to promote continual improvement of the environmental management system. "We will clearly define measures to achieve the targets and conduct various audits, etc., in order to further improve and enhance our management systems designed to safeguard the environment and reduce the environmental impact of our products. We will also confirm the validity of our environmental policy and all management systems through a management review conducted at the end of each year."
- Prevent community from pollution not only during normal operations but also in emergencies such as abnormal circumstances and accidents. "If we should negatively impact the global environment or if a negative impact is anticipated, or when an accident or other problem has occurred through use of our product, we will implement an appropriate solution sincerely in a timely manner and undertake to prevent any recurrence of the problem. We will also conduct emergency drills periodically so that the procedures to be taken are communicated and clearly understood by all employees."
- Make all company associates aware of the basic concepts about environmental management and train them to conduct activities in accordance with the environmental policy. "We will develop an educational plan and educate all people working for the organization regarding the safeguarding of the environment and environmental impact of our products, to raise their awareness of the environment and urge them to act in line with our environmental policy."
- Make the policy readily accessible to the public. "We will distribute 'Environmental Policy Cards' and use the website to actively disclose information regarding our environmental management activities both within and outside the company, and then use the feedback received in our future activities.
Environmental Management System
Based on the Mid-Term Environmental Management Plan developed according to the Environmental Policy, each business site is developing its own targets reflecting the local environment and specific business operations of the site while examining specific measures to achieve these targets, in order to reduce the environmental impact. The implementation condition of these measures is checked by the Environmental Management Committee and through internal environmental audits. The audit results are reported at the Management Review Meeting, etc., and if necessary, a management system review is conducted and the review results reflected in activities in the next year onward. The details and outcomes of activities carried out each year are compiled by the Administration Office (General Affairs Unit, Material Manufacturing Department's General Affairs Section) into an Environmental Report," which is published for the employees and the general public. These steps are carried out cyclically and continuously to increase the completeness of our systems and further enhance the level of target achievement.
|
Overview of Environmental Management 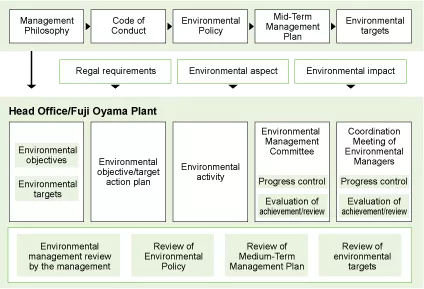
|
Number of environmentally friendly products developed (pieces) 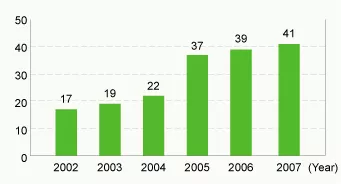
|
Philosophy



Background of Foundation
Eighty-six years ago, three youths who had just graduated from Tokyo Imperial University (currently the University of Tokyo) with a degree in applied chemistry got together and set up a small research company in a 50m2 space in Ikebukuro, Tokyo. In those days, all dental materials were imported. Brimming with aspirations, the three young entrepreneurs named their company "GC (General Chemical)".
The following year, GC introduced its first product, "Standard Cement," but it flopped in the marketplace. However, this experience provided a great learning ground for the three founders, who realized the "importance of making products by thinking from the customer's standpoint" and fostered an "uncompromising commitment to quality." Three years later, they created "Crystalline Cement," which offered excellent quality comparable to imported materials. Partly as a reminder of their initial failure, the three designated February 11--the date of the first product's launch--as the anniversary of the company. The dedication of the founders to making quality products has been kept alive through generations of GC men and women, and it continues to drive the company's growth.
Based on the Teachings of Semui
Semui is a notion that constitutes the core of the so-called Kannon Sutra. To explain this concept using modern terms, it is a way of life by which one rids oneself of selfish thoughts and puts himself/herself in the situation of others. Doing everything by thinking from the standpoint of others ultimately helps the individual live a good life.
Kiyoshi Nakao, one of the founders of GC, instituted this company's motto based on the teaching of "Semui" following the failure of "Standard Cement:" "A true product is a product of interdependence, made by ridding oneself of selfish thoughts and thinking from the standpoint of others." All employees at GC act as members of a big family, where they remove themselves from egocentric thoughts and respect their fellows, or "Nakama." They also strive to think from the customer's standpoint and make true products by placing a priority on the value these products provide for customers.
Commitment of GC
GC has stubbornly maintained the devotion of its founders to products, its employees and society. In 1981 we introduced our unique system of total quality control called GQC (GC's Quality Control). Subsequently, GQC was expanded into the GQM (GC's Quality Management) activity, which is now driving company-wide efforts to contribute to society through its products. We at GC are committed to achieving excellent quality, and this commitment is firmly rooted in all employees working in all departments relating to research, production sales, etc. Indeed, this thinking is an important cultural asset of GC. By remaining true to this commitment, GC will continue to play a part in society through dental products in order to make the twenty-first century the "Century of Health."
Global Network
Nationwide Network Offering Finely Tailored Service
GC Corporation has built up a Japan-wide network of distributorships, as well as a core establishment of branches, sales offices and service centers. Creation of a substantial before- and after-sales service system is also well underway, using as combination of DR activities (Dental Representative carried out by "GC Man" and "GC Lady") and machine maintenance and service centers.
GC is also using its "Green System" (based on the world-leader in ERP software packages, R/3) to facilitate shared access to the company's database information in real time and so expedite selectivity between distribution and sales. As a result, developments in logistics that deploy IT have led to data centralization and to center-based delivery of product. By utilizing its nation-wide dealer network, GC is able to deliver fresh product to its users with optimal ensure the safety and effectiveness of the products that people receive.
GC's Worldwide Sales and Production Network
GC manufactures some 600 types of products, which are sold in over 100 countries around the world. Due to the existence of differing needs in different overseas markets, GC has very deliberately localized its European, America and Asian operations. It is losing no time in optimizing its on-site activities, while at the same time fulfilling its obligation as a local enterprise by taking an active part in dental industry associations in each country. In terms of its daily operations, GC is not only using contract distributorships to expand its sales network, but is also developing its user-oriented activities through close ties with key opinion leaders in key markets.
In this way, GC is embracing the priority markets of Europe and the United States - markets with comparatively large market volumes - but manufacturing in optimum locations around the world, thereby accommodating market demand while building a presence in the Chinese and Indian markets - markets with firm expectations of growth. Its three main production centers have all acquired ISO13485 certification, and the Company is becoming increasingly well known in the global marketplace for the quality of its products.
GC aims to bring product reliability and product quality of a global standard to the people of the world, thereby making the 21st century "the century of health."
Research & Development






GC is Constantly Actively Engaged in Research and Development in Harmony with Both Social and Natural Environments
Based on the GC's corporate philosophy, "Semui; respecting the customer's point of view", all the products GC offers are developed solely for the sake of people. As dental materials are often applied directly into the body, it is essential that they should be safe, reliable and effective. GC engages in research and development with its various advanced analytic equipments to develop materials including inorganic, organic or metallic to make most of the property of each type; continues the best efforts for accumulation and practical use of core technologies; thus delivers the world's No.1 quality products to markets. Furthermore, it combines the highest technology for material development and equipment design with information technologies so that it could keep advancing also in the field of CAD/CAM systems as well as implant systems to extend their applications.
Its development of state-of-the-art technology, including regenerative therapy utilizing cell and tissue culture, such as the regeneration of alveolar bone and genetic treatments based on human genome research, allows GC to provide evidence based materials, equipments and information, in co-operation with the enterprises and research organizations both domestic and overseas which possess the utmost technologies in respective fields. In the area of dental equipments, as well, our innovative technologies allow alleviation of burdens for both dental staffs and patients and enable us to advocate comfortable therapeutic circumstances. In addition, our efforts are also assigned to achieve provision of environment-friendly products using the packages with minimized wastes.
We believe that GC's mission is to respond to the changing needs of the global markets and to develop new products in harmony with both social and natural environments. There are unlimited opportunities in dentistry in the drive to build truly healthy and longliving communities. We will continue to place a high priority on R&D in our new R&D Building, based on communication through colleagues, so that we will open the door to a brilliant future.
Foundation Nakao for Worldwide Oral Health

On September 21, 2018, Foundation Nakao for Worldwide Oral Health was established in Luzern, Switzerland. This was made possible by Mr. Makoto Nakao, former Chairman of GC Corporation, who after 42 years at the helm of the company donated his privately-owned company shares to support this noble initiative.
Foundation Nakao supports academic research and clinical studies contributing to its founding goal, which is the improvement of oral health and subsequent raised quality of life of all people in the world. Key oral health research areas that address Minimum Intervention Dentistry, Oral Health in Aging Populations and the 8020 movement, Tooth Function, the prevention of Oral Frailty and Dental IQ.
The management board of Foundation Nakao boasts a team of distinguished dental professionals from four continents: Europe, America, Australia, and Asia. They are Professor Reinhart Hickel, Professor Clark Stanford, Professor Marco Ferrari, Professor Eric Reynolds, Professor Keiichi Sasaki and Dr Kiyotaka Nakao.
For more information, please visit: https://www.foundation-nakao.com.